Member LoginDividend CushionValue Trap |
Recent Events Concerning Johnson & Johnson
publication date: Jun 18, 2020
|
author/source: Callum Turcan
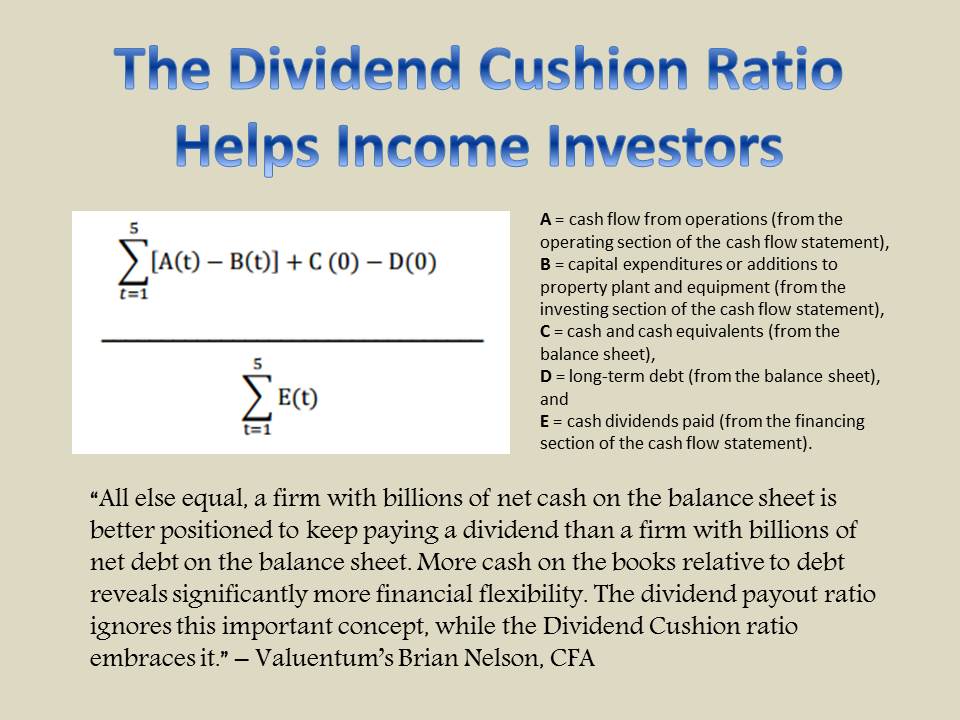
Image Source: Johnson & Johnson – First Quarter of 2020 IR Earnings Presentation By Callum Turcan We include Johnson & Johnson (JNJ) as a top-weighted holding in the Dividend Growth Newsletter portfolio and as a medium-weighted holding in the Best Ideas Newsletter portfolio. The firm’s Dividend Cushion ratio sits at a solid 2.1, and please note that this forward-looking dividend coverage ratio factors in our expectations that Johnson & Johnson will grow its per share dividend by mid-single-digits annually over the coming years. Johnson & Johnson earns a “GOOD” Dividend Safety rating and an “EXCELLENT” Dividend Growth rating, with shares of JNJ yielding ~2.8% as of this writing. In our view, Johnson & Johnson’s strong balance sheet and high quality cash flow profile provide it with the financial strength to ride out the storm created by the ongoing coronavirus (‘COVID-19’) pandemic with its current dividend policy and financials intact. COVID-19 Vaccine Update On June 10, Johnson & Johnson put out a press release noting that the firm had “accelerated the initiation of the Phase 1/2a first-in-human clinical trial of its investigational SARS-CoV-2 vaccine, Ad26.COV2-S, recombinant. Initially scheduled to begin in September, the trial is now expected to commence in the second half of July.” We appreciate the news given the urgent need for a COVID-19 vaccine and considering some of Johnson & Johnson’s peers are either already in the process of running human clinical trials, such as Moderna Inc (MRNA) [more on that here], or will soon be doing so. Johnson & Johnson’s Janssen pharmaceutical unit is working with the Biomedical Advanced Research and Development Authority (‘BARDA’) which operates as an office of the US Department of Health & Human Services (‘HHS’). Here are some additional highlights on the upcoming human clinical trials from Johnson & Johnson’s press release: The randomized, double-blind, placebo-controlled Phase 1/2a study will evaluate the safety, reactogenicity (response to vaccination), and immunogenicity (immune response) of the investigational SARS-CoV-2 vaccine, Ad26.COV2-S, recombinant in 1045 healthy adults aged 18 to 55 years, as well as adults aged 65 years and older. The study will take place in the U.S. and Belgium. The Company is in discussions with the National Institutes of Allergy and Infectious Diseases with the objective to start the Phase 3 SARS-CoV-2 vaccine, Ad26.COV2-S, recombinant, clinical trial ahead of its original schedule, pending outcome of Phase 1 studies and approval of regulators. Please note that Johnson & Johnson is also aggressively ramping up its manufacturing capacity and aims to supply 1 billion doses of the potential vaccine in 2021, assuming the vaccine is both effective and safe. Recently, the European Union has been in touch with Johnson & Johnson about securing supplies of its potential vaccine. That reportedly could include reserving some of the expected vaccine supplies or purchasing those expected vaccine supplies upfront. Reportedly, a deal is expected to be announced in the coming weeks, though nothing is for certain at this stage. Back in the middle of May, we published an article highlighting the various vaccines and cures under development that could be effective at dealing with COVID-19, which we encourage members to check out here. We covered Johnson & Johnson’s COVID-19 vaccine efforts in that note as well. Baby Power Sales to End After selling some form of the talc-based Johnson’s Baby Powder since the 1890s, Johnson & Johnson is throwing in the towel and discontinuing sales in the US and Canada. This decision was likely in part due to the company reporting ongoing weakness in sales of the product line (‘Baby Care’ was the only sub-segment within the firm’s ‘Consumer Health’ segment to post a year-over-year decline in sales in the first quarter of 2020). In our view, the biggest reason the company is cancelling its Johnson’s Baby Powder product line in the US and Canada is due to ongoing lawsuits alleging the talc-based product resulted in some consumers getting cancer. This was allegedly due to the talc Johnson & Johnson sourced being contaminated with asbestos (asbestos, a carcinogen, is sometimes located near talc deposits in nature). We covered the issue in detail here and Johnson & Johnson’s response here. Please note Johnson & Johnson has emphatically denied the allegations and the firm has contracted third-party labs to prove its Johnson’s Baby Powder product does not contained asbestos (so far, those labs have shown that Johnson’s Baby Powder does not contain asbestos as alleged by the US Food and Drug Administration last year). We will keep our members informed on the issue as it develops. Concluding Thoughts We continue to like Johnson & Johnson in the Best Ideas Newsletter and Dividend Growth Newsletter portfolios. Our fair value estimate for shares of JNJ stands at $148 and there is room for meaningful upside here as the top end of our fair value estimate range sits at $178 per share of JNJ. Once global health authorities begin to get a handle on the ongoing pandemic, that should materially improve Johnson & Johnson’s outlook. The firm’s Consumer Health and ‘Pharmaceuticals’ segments performed very well in the first quarter of this year, but its ‘Medical Devices’ segment did not as elective surgeries were postponed. Once elective surgeries resume in earnest, Johnson & Johnson’s financial performance should improve materially. We covered the firm’s latest quarterly earnings report in this article here, which we encourage our members to check out that piece if you have not done so already. On a final note, Johnson & Johnson increased its quarterly dividend by 6% sequentially in April 2020, highlighting management’s confidence in the firm’s future financial performance. ----- Medical Devices Industry – EW ISRG MDT VAR WAT ZBH Health Care Services Industry – DVA EHC HCA UNH UHS Pharmaceuticals (Big) Industry – ABT ABBV AMGN AZN BMY LLY GSK MRK NVS NVO PFE SNY Pharmaceuticals (Biotech/Generic) Industry – ALXN AGN BHC BIIB BMRN GILD MYL REGN TEVA VRTX ZTS Household Products Industry – CHD CLX CL ENR HELE JNJ KMB PG Related: XLV, STT, MNK, ENDP, CAH, MCK, ABC, WMT, RAD, CVS, IBB Related (vaccine/treatment): SRNE, MRNA, INO, NVAX, BNTX, APDN, VXRT, TNXP, EBS, PFE, JNJ, DVAX, IMV, IBIO, REGN, SNY, GSK, ABBV, TAK, HTBX, SNGX, PDSB ----- Valuentum members have access to our 16-page stock reports, Valuentum Buying Index ratings, Dividend Cushion ratios, fair value estimates and ranges, dividend reports and more. Not a member? Subscribe today. The first 14 days are free. Callum Turcan does not own shares in any of the securities mentioned above. The Health Care Select Sector SPDR ETF (XLV) and Johnson & Johnson (JNJ) are both included in Valuentum’s simulated Best Ideas Newsletter and Dividend Growth Newsletter portfolios. Both the simulated Best Ideas Newsletter and Dividend Growth Newsletter portfolios include a SPDR S&P 500 ETF Trust (SPY) put option holding with a $295 per share strike price that expire on August 21, 2020. Some of the other companies written about in this article may be included in Valuentum's simulated newsletter portfolios. Contact Valuentum for more information about its editorial policies. |


1 Comments Posted Leave a comment