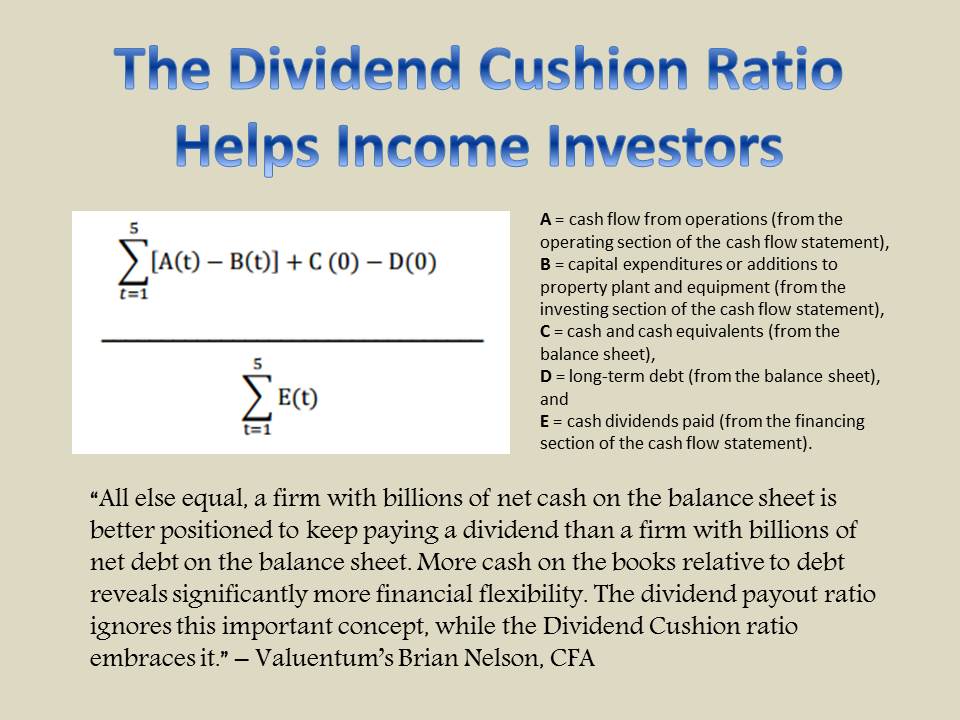
Member LoginDividend CushionValue Trap |
Excited By COVID-19 Vaccine Candidates
publication date: May 18, 2020
|
author/source: Callum Turcan and Brian Nelson, CFA
Image Shown: The race is on to find a cure, or better yet a vaccine, for COVID-19. Image Source: Pfizer Inc – First Quarter 2020 Earnings IR Presentation Key Takeaways The race for a COVID-19 cure and vaccine is rapidly evolving with a lot of exciting press releases being put forth. Gilead has taken the lead with a viable treatment, Sorrento is working toward a cure, and it seems most all of big pharma and biotech is racing to find a vaccine, from Johnson & Johnson to Sanofi/GSK and beyond. Though the evaluation of the full data set from a Phase 2 clinical trial means a lot more than the evaluation of a limited set of data from a Phase 1 clinical trial, we think COVID-19 is on the run as modern medicine pushes forward. We’re reiterating our bullish take on the markets today, as we believe that the Fed will do anything and everything to keep this market moving higher, meaning stocks may remain divorced both from economic data and even virus data for some time as they continue to climb. We continue to point to ideas in the Best Ideas Newsletter portfolio, Dividend Growth Newsletter portfolio, High Yield Dividend Newsletter portfolio and Exclusive publication. Our top 10 capital appreciation ideas and dividend growth ideas amid COVID-19, respectively, can be found at the following link, “Valuentum's COVID-19 Ideas Have Outperformed Significantly.” As we walk through a ‘who’s who’ as it relates to COVID-19 vaccine candidates, we maintain our view that investors may be facing a “win-win” situation as we outlined in our piece, “Stay Optimistic. Stay Bullish. I Am.” We remain unequivocally bullish on stocks for the long run. By Callum Turcan and Brian Nelson, CFA On May 15, President Donald Trump launched “Operation Warp Speed,” and the race to find a vaccine for coronavirus (‘COVID-19’) that has infected millions and killed hundreds of thousands worldwide is on. Much like in the spirit of the Manhattan Project, a massive undertaking during World War II to beat the Nazis in a race to develop the world’s first nuclear weapons, “Operation Warp Speed” is aimed at developing a vaccine for COVID-19 as quickly as possible, with an aim before the end of the year, an achievement that would be unprecedented in modern medicine. According to reports, those heading the new initiative have been working “through roughly 100 vaccine candidates from all over the world and have identified more than 14 believed to be the most promising.” To speed up vaccine availability, the United States will invest in the top vaccine candidates even before they are approved, so that once a vaccine is signed off on by the FDA, it can be rapidly rolled out across the world. Many pharma and biotech companies have already announced they will be making large volumes of their vaccine candidates before approval in an effort to get a viable vaccine to market without delay. We continue to be encouraged by the number of shots on goal for COVID-19 treatments and vaccine candidates. On May 1, the FDA approved the emergency use of Gilead’s (GILD) remdesivir for the treatment of COVID-19 infection after the drug showed improved recovery time in patients with severe symptoms of the disease. Top national health official Dr. Anthony Fauci said that remdesivir is the standard of care, noting the drug has been proven to block the virus. Remdesivir may be just the first step in potential advancements for the treatment for COVID-19, however, as both big pharma and biotech work tirelessly to save lives and develop a vaccine for the deadly virus. Though a cure or vaccine for COVID-19 may still be some time away, the accelerated progress in just the past few months has been remarkable. As we walk through a ‘who’s who’ as it relates to COVID-19 vaccine candidates, we maintain our view that investors may be facing a “win-win” situation as we outlined in our piece, “Stay Optimistic. Stay Bullish. I Am.” We’re reiterating our bullish take on the markets today, as we believe that the Fed will do anything and everything to keep this market moving higher, meaning stocks may remain divorced both from economic data and even virus data for some time as they continue to climb. With moral hazard running rampant in the form of index investing and modern portfolio theory, stocks may once again reach new highs as the Fed/Treasury may have no choice but to back fiduciaries employing such investment strategies. Let’s now get into the most promising vaccine candidates for COVID-19. Johnson & Johnson On March 30, Johnson & Johnson’s (JNJ) Janssen subsidiary announced that it teamed up with the Biomedical Advanced Research and Development Authority (‘BARDA’), a division of the Office of the Assistant Secretary for Preparedness and Response (‘ASPR’) which is a part of the U.S. Department of Health and Human Services (‘HHS’), and committed to invest $1.0 billion toward developing a COVID-19 vaccine. In January 2020, Johnson & Johnson began working with Beth Israel Deaconess Medical Center (part of Harvard Medical School) to locate a viable vaccine candidate, and now Johnson & Johnson is pivoting towards the next phases of development. The partnership has identified one leading vaccine candidate and two backups and expects to begin human clinical trials by September 2020. Here’s an excerpt from the March 30 press release: Through collaborations with scientists at multiple academic institutions, the vaccine constructs were then tested to identify those with the most promise in producing an immune response in preclinical testing. Based on this work, Johnson & Johnson has identified a lead COVID-19 vaccine candidate (with two back-ups), which will progress into the first manufacturing steps. Under an accelerated timeline, the Company is aiming to initiate a Phase 1 clinical study in September 2020, with clinical data on safety and efficacy expected to be available by the end of the year. This could allow vaccine availability for emergency use in early 2021. For comparison, the typical vaccine development process involves a number of different research stages, spanning 5 to 7 years, before a candidate is even considered for approval. Consistent with “Operation Warp Speed,” the goal is to simultaneously discover a viable and safe vaccine while also ramping up manufacturing capacity of the potential vaccine. In the past, scaling up production capacity would come after proving the therapeutic candidate is viable, but as with others that are racing to develop a vaccine, Johnson & Johnson wants to get ahead of the curve by already having the means to produce hundreds of millions of doses by the start of next year at its and its partnership’s disposal. To do so, Johnson & Johnson partnered up with Emergent Biosolutions Inc (EBS) through a deal announced on April 23 to assist in scaling up its manufacturing capacity to produce its potential COVID-19 vaccine. Johnson & Johnson is assisting Emergent through Janssen’s AdVac and PER.C6 vaccine technology, which were used in the past to develop vaccine candidates to treat Ebola, Zika, RSV, and HIV. Here’s a key excerpt from the April 23 press release: Under the terms of this manufacturing agreement, Johnson & Johnson is investing to expand drug substance capacity related to the vaccine candidate. Emergent will provide drug substance manufacturing services with its molecule-to-market CDMO [contract development and manufacturing organization] offering, beginning in 2020, and will also reserve operations capacity to potentially support commercial manufacturing of Johnson & Johnson’s COVID-19 vaccine candidate leveraging Janssen’s proven AdVac® and PER.C6® technologies beginning in 2021. The objective is to produce over one billion doses of the vaccine, if not more, in a compressed timeframe, and we appreciate Johnson & Johnson’s commitment. As more information becomes available with respect to this endeavor, we’ll have more to say on Johnson & Johnson’s push to be the first to develop a COVID-19 vaccine. The move isn’t likely to be needle-moving to Johnson & Johnson’s financials (i.e. revenues) in a direct way (indirectly, discovering a vaccine could help by stabilizing the global economy), it could generate material goodwill for the firm and improve its brand in the eyes of consumers around the world, on top of saving potentially millions of lives. We continue to like shares of JNJ as a top-weighted idea in the Dividend Growth Newsletter portfolio and as a moderately-weighted idea in our Best Ideas Newsletter portfolio. Pfizer Pfizer Inc (PFE) is addressing the COVID-19 pandemic in several ways. For starters, Pfizer is working with BioNTech SE (BNTX) to develop BioNTech’s mRNA-based coronavirus vaccine, with the partnership announcing a letter of intent to collaborate on the COVID-19 vaccine candidate on March 17. Clinical trials on human beings were up and running in both the US and Germany by early May, and furthermore, “the companies estimate that there is potential to supply millions of vaccine doses by the end of 2020 subject to technical success of the development program and approval of regulatory authorities.” Pfizer and BioNTech, if successful, aim to supply “hundreds of millions of doses in 2021” by ramping up their combined manufacturing capacity. On April 22, the partnership announced it had received approval from the German regulator Paul-Ehrlich-Institut to launch a Phase 1/2 clinical trial. By early May, the partnership had begun to provide doses of the vaccine US clinical trial participants as part of their Phase 1/2 clinical trial and had already begun to dose German clinical trial participants (from a May 5 press release): [The partnership between Pfizer and BioNTech] announced today that the first participants have been dosed in the U.S. in the Phase 1/2 clinical trial for the BNT162 vaccine program to prevent COVID-19. The trial is part of a global development program, and the dosing of the first cohort in Germany was completed last week.. The Phase 1/2 study is designed to determine the safety, immunogenicity and optimal dose level of four mRNA vaccine candidates evaluated in a single, continuous study. The dose level escalation portion (Stage 1) of the Phase 1/2 trial in the U.S. will enroll up to 360 healthy subjects into two age cohorts (18-55 and 65-85 years of age). The first subjects immunized in Stage 1 of the study will be healthy adults 18-55 years of age. Older adults will only be immunized with a given dose level of a vaccine candidate once testing of that candidate and dose level in younger adults has provided initial evidence of safety and immunogenicity. Sites currently dosing participants include NYU Grossman School of Medicine and the University of Maryland School of Medicine, with the University of Rochester Medical Center/Rochester Regional Health and Cincinnati Children’s Hospital Medical Center to begin enrollment shortly. Pfizer and BioNTech have located four different COVID-19 vaccine candidates, and each candidate is using “a different combination of mRNA format and target antigen” which is similar to what some other companies are pursuing. Simultaneously, Pfizer and BioNTech are getting their combined global manufacturing presence ready to enable widespread production of a COVID-19 vaccine candidate should their efforts prove successful. Merck While a giant in the pharmaceutical space, Merck & Co Inc (MRK) has been slower to launch a major push to develop a COVID-19 vaccine than its peers. During its first-quarter 2020 earnings report published April 28, the firm announced it was “assessing (its) available antiviral candidates” and “in advanced discussions with multiple groups, focusing on three different viral vaccine platforms” keeping in mind that “details of those collaborations will be announced when the necessary arrangements are finalized” which as of this writing, had yet to be announced. Merck is participating with various research groups and government agencies, including the Accelerating COVID-19 Therapeutic Interventions and Vaccines (‘ACTIV’) consortium which is being led by the National Institutes of Health (‘NIH’), to find a way to contain or ideally eradicate the virus. We’ll see what Merck comes up with in the coming months. Moderna Small-cap biotech Moderna (MRNA) was awarded ~$0.5 billion from BARDA to accelerate the development of its leading COVID-19 vaccine candidate (‘mRNA-1273’) through a deal announced back in April 16. Here’s what the press release had to say: Under the terms of the agreement, BARDA will fund the advancement of mRNA-1273 to FDA licensure. A Phase 1 study of mRNA-1273 is being conducted by the National Institutes of Health (NIH). The Phase 1 open-label study, which began on March 16, 2020 has completed enrollment of the original study: 45 healthy adult volunteers ages 18 to 55 years in three dose cohorts (25 µg, 100 µg and 250 µg). The NIH recently amended the Phase 1 protocol to include an additional six cohorts: three cohorts of older adults (ages 56 -70) and three cohorts of elderly adults (age 71 and above). Enrollment for these cohorts is ongoing. As a prophylactic vaccine candidate, the goal of mRNA-1273 is to introduce antigens into the human body so the immune system will respond and create antibodies. If effective, the vaccinated human becomes immune to the associated illness. Moderna noted the following in its first-quarter 2020 earnings press release, published May 7: Novel coronavirus (SARS-CoV-2) vaccine (mRNA-1273): The U.S. Food and Drug Administration (FDA) completed its review of the Company’s Investigational New Drug (IND) application for its novel coronavirus (SARS-CoV-2 or COVID-19) vaccine candidate (mRNA-1273) allowing it to proceed to the Phase 2 study. A 600 participant Phase 2 study is expected to begin shortly. The Company is finalizing the protocol for the Phase 3 study, which is expected to begin in the early summer of 2020. Moderna is pushing ahead with Phase 2 of its clinical trials as it gets closer to finishing Phase 1, which is a promising sign. On May 12, the US FDA granted Moderna’s mRNA-1273 COVID-19 vaccine candidate a ‘Fast Track’ designation (from Moderna’s press release announcing the Fast Track designation): Fast Track is designed to facilitate the development and expedite the review of therapies and vaccines for serious conditions and fill an unmet medical need. Programs with Fast Track designation may benefit from early and frequent communication with the FDA, in addition to a rolling submission of the marketing application… About the Phase 2 Study Moderna has received initial feedback from the FDA on the design of the planned Phase 2 study, which will evaluate the safety, reactogenicity and immunogenicity of two vaccinations of mRNA-1273 given 28 days apart. The Company intends to enroll 600 healthy participants across two cohorts of adults ages 18-55 years (n=300) and older adults ages 55 years and above (n=300). Each participant will be assigned to receive placebo, a 50 μg or a 250 μg dose at both vaccinations. Participants will be followed through 12 months after the second vaccination. On May 18, Moderna announced “positive” data from its “interim” Phase 1 clinical trial covering mRNA-1273 (keeping in mind this is only Moderna’s interpretation of a limited data set and doesn’t cover the data from all of the clinical trial’s participants) and as it relates to future clinical trial phases the firm noted: Based on the interim Phase 1 data, the Moderna-led Phase 2 study will be amended to study two dose levels, 50 µg and 100 µg, with the aim of selecting a dose for pivotal studies. The NIAID-led Phase 1 study is being amended to include a 50 µg dose level cohort across each of the three age groups. Moderna anticipates the dose for the Phase 3 study to be between 25 µg and 100 µg and expects Phase 3 trial initiation in July, subject to finalization of the clinical trial protocol. Moderna’s mRNA-1273 COVID-19 vaccine candidate is at or near the front of the pack, and we’ll be keeping a close eye on future developments. It appears that the Phase 2 clinical trial should commence relatively soon (planning on the study is ongoing) with the Phase 3 clinical trial set to begin in July, according to information provided in Moderna’s latest press release. Please note that the Phase 1 clinical trial only included 45 participants while the Phase 2 clinical trial will cover 600 participants, according to CNBC, making it far more meaningful on a statistical basis. Sanofi and GlaxoSmithKline French pharma giant Sanofi (SNY) and GlaxoSmithKline (GSK) announced April 14 that the combo would be joining forces in working together to develop a viable vaccine candidate for COVID-19. Here is what the duo had to say: Sanofi will contribute its S-protein COVID-19 antigen, which is based on recombinant DNA technology. This technology has produced an exact genetic match to proteins found on the surface of the virus, and the DNA sequence encoding this antigen has been combined into the DNA of the baculovirus expression platform, the basis of Sanofi’s licensed recombinant influenza product in the US. GSK will contribute its proven pandemic adjuvant technology to the collaboration. The use of an adjuvant can be of particular importance in a pandemic situation since it may reduce the amount of vaccine protein required per dose, allowing more vaccine doses to be produced and therefore contributing to protect more people. The team expects a vaccine to enter clinical trials in the back half of 2020, and if they are successful in their endeavor, the company said a vaccine for COVID-19 would be available by the second half of next year. We’re not betting against two of the world’s largest vaccine companies. Sanofi could have 600 million doses of a COVID-19 vaccine by next year. Sorrento Therapeutics Another very interesting player in the race for a COVID-19 treatment is Sorrento Therapeutics (SRNE), which announced on May 15 “that its anti-SARS-CoV-2 antibody, STI-1499, demonstrated 100% inhibition of SARS-CoV-2 virus infection in an in vitro virus infection experiment at a very low antibody concentration” and this news saw shares of SRNE shoot up triple-digits during normal trading hours that day. Sorrento Therapeutics’ goal is to develop an antibody cocktail to fight the coronavirus, and while this isn’t the same as a COVID-19 vaccine, STI-1499 could prove to be a powerful stop gap measure. The STI-1499 antibody was proven effective at neutralizing the coronavirus in lab experiments; now, the next step is to see how the therapy performs during animal trials, which if successful will be followed up by human trials that may commence this upcoming July, according to The San Diego Union-Tribune. Here’s what Sorrento Therapeutics had to say in the May 15 press release: Among the antibodies showing neutralizing activity, one antibody stood out for its ability to completely block SARS-CoV-2 infection of healthy cells in the experiments. STI-1499 completely neutralized the virus infectivity at a very low antibody dose, making it a prime candidate for further testing and development. Initial biochemical and biophysical analyses also indicate STI-1499 is a potentially strong antibody drug candidate. Sorrento has determined STI-1499 will likely be the first antibody in the antibody cocktail (COVI-SHIELD™) it is developing, as recently announced. STI-1499 is also expected to be developed as a stand-alone therapy, (COVI-GUARD™) because of the high potency it has exhibited in experiments to date. Sorrento plans to request priority evaluation and accelerated review from regulators to determine the best pathway to make any potential treatment available as soon as possible. While the world waits for a COVID-19 vaccine to be widely available, Sorrento Therapeutics could provide the means to end the “cocooning” of households by providing a viable COVID-19 therapy option, keeping in mind the San Diego-based company would likely need help in ramping up its manufacturing capabilities. Other Notables Arcturus Therapeutics (ARCT): “Arcturus Reports Additional Supportive Preclinical Data for its COVID-19 Vaccine Candidate (LUNAR-COV19)” iBio (IBIO): “iBio Estimates ~500 Million Dose Capacity for COVID-19 Vaccine from its FastPharming Facility” Dynavax Technologies (DVAX): “Valneva and Dynavax Announce Collaboration to Advance Vaccine Development for COVID-19” Inovio (INO): “INOVIO Completes Enrollment in the Phase 1 U.S. Trial of INO-4800 for COVID-19 DNA Vaccine; Interim Results Expected in June” Novavax (NVAX): “Novavax to commence COVID-19 vaccine trial with Nucleus Network” Soligenix (SNGX): “Soligenix Announces Exclusive Licensing Agreement for Novel Vaccine Adjuvant from BTG Specialty Pharmaceuticals” Vaxart (VXRT): “Vaxart Announces Additional Positive Pre-Clinical Data for its Oral COVID-19 Vaccine Program” Concluding Thoughts The race for a COVID-19 cure and vaccine is rapidly evolving with a lot of exciting press releases being put forth. Gilead has taken the lead with a viable treatment, Sorrento is working toward a cure, and it seems most all of big pharma and biotech is racing to find a vaccine, from Johnson & Johnson to Sanofi/GSK and beyond. Though the evaluation of the full data set from a Phase 2 clinical trial means a lot more than the evaluation of a limited set of data from a Phase 1 clinical trial, we think COVID-19 is on the run as modern medicine pushes forward. We remain unequivocally bullish on stocks for the long run, “Stay Optimistic. Stay Bullish. I Am.” ----- Medical Devices Industry – EW ISRG MDT VAR WAT ZBH Health Care Services Industry – DVA EHC HCA UNH UHS Pharmaceuticals (Big) Industry – ABT ABBV AMGN AZN BMY LLY GSK MRK NVS NVO PFE SNY Pharmaceuticals (Biotech/Generic) Industry – ALXN AGN BHC BIIB BMRN GILD MYL REGN TEVA VRTX ZTS Household Products Industry – CHD CLX CL ENR HELE JNJ KMB PG Related: XLV, STT, MNK, ENDP, CAH, MCK, ABC, WMT, RAD, CVS, IBB Related (vaccine/treatment): SRNE, MRNA, INO, NVAX, BNTX, APDN, VXRT, TNXP, EBS, PFE, JNJ, DVAX, IMV, IBIO, REGN, SNY, GSK, ABBV, TAK, HTBX, SNGX, PDSB Tickerized for our healthcare and biotech ETF coverage. ----- Valuentum members have access to our 16-page stock reports, Valuentum Buying Index ratings, Dividend Cushion ratios, fair value estimates and ranges, dividend reports and more. Not a member? Subscribe today. The first 14 days are free. Callum Turcan does not own shares in any of the securities mentioned above. Brian Nelson owns the SPY and SCHG. The Health Care Select Sector SPDR ETF (XLV) and Johnson & Johnson (JNJ) are both included in Valuentum’s simulated Best Ideas Newsletter and Dividend Growth Newsletter portfolios. Some of the other companies written about in this article may be included in Valuentum's simulated newsletter portfolios. Contact Valuentum for more information about its editorial policies. |

0 Comments Posted Leave a comment